tin electron configuration|full electron configuration of platinum : Manila Tin is a soft, malleable, ductile and highly crystalline silvery-white metal. When a bar of tin is bent a crackling sound known as the "tin cry" can be heard from the twinning of the crystals. This trait is shared by indium, cadmium, zinc, and mercury in its solid state. Tin melts at about 232 °C (450 °F), the lowest in group 14. The melting point is further lowered to 177.3 °C (351.1 °F) for 11 nm particles. SM Aura Premier Cinema 1, SM Aura Premier, Taguig. Movies TV Food & Drink Shops & Services. Tickets Menu. Get Tickets. Ayala Malls Cinemas Robinsons Movieworld Megaworld Lifestyle Malls. Search. Popular Malls Glorietta Robinsons Galleria South SM Aura Premier SM Mall of Asia SM North EDSA. Popular Theaters
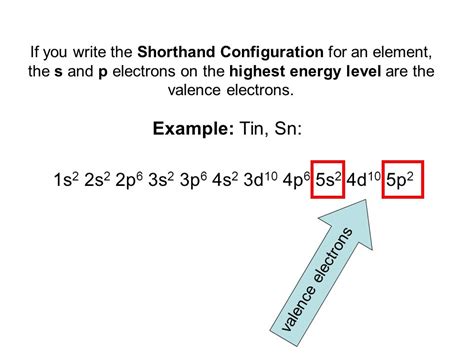
tin electron configuration,Electron Configuration of Tin. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (II) compounds with its two unpaired p-electrons. In the three dimensional .Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. .
Tin is a soft, malleable, ductile and highly crystalline silvery-white metal. When a bar of tin is bent a crackling sound known as the "tin cry" can be heard from the twinning of the crystals. This trait is shared by indium, cadmium, zinc, and mercury in its solid state. Tin melts at about 232 °C (450 °F), the lowest in group 14. The melting point is further lowered to 177.3 °C (351.1 °F) for 11 nm particles.
Tin is a post-transition metal with the symbol Sn and atomic number 50, and has two possible oxidation states, +2 and +4. Learn about its physical and chemical . The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons within the atom. This configuration .
Full electron configuration of tin: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. indium ← tin → antimony. Tin, complete electron configuration.

Tin Electron Configuration: Tin is a chemical element that has the symbol Sn (It is taken from the Latin word tannum ). The atomic number of Tin is 50. It is one of the post-transition metals in group 14 of .
Introduction. Historical Background. Physical Properties. Chemical Properties. Electron Configuration. Oxidation States. Common Compounds. Notable Chemical Reactions. .
Tin is a post-transition metal with atomic number 50 and 50 electrons in its neutral atom. The electron configuration of tin is [Kr] 4d10 5s2 5p2, which means it has 10 valence electrons in its outermost shell. . To write the configuration for the Tin (Sn) and the Tin ions, first we need to write the electron configuration for just Tin (Sn). We first need to find the.
The electronic configuration of Tin will be 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. How do you write the electron configuration for Tin? The electronic configuration of Tin will be 1s2 2s2 2p6 3s2 3p6 3d10 .Electron configuration 4d 10 5s 2 5p 2: Electrons per shell: 2, 8, 18, 18, 4: . Tin is a chemical element; it has symbol Sn (from Latin stannum) and atomic number 50. A silvery-colored metal, tin is soft enough to be cut .
To find the number of valence electrons for Tin (Sn) we need to look at its electron configuration. This is necessary because Sn is a transition metal (d bl.
Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. The electron configuration for the first 10 elements. H .⬆️⬆️⬆️ Tin 的 Electron 配置是 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2。 锡是元素周期表中的化学元素,属于族

Protons and Neutrons in Tin. Tin is a chemical element with atomic number 50 which means there are 50 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
full electron configuration of platinum Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic structure.The chemical symbol for Tin is Sn. Electron Configuration and Oxidation States of Tin. Electron configuration of Tin is [Kr] 4d10 5s2 5p2. Possible oxidation states are +2,4. Electron Configuration. The .
Introduction. The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. In doing so, we obtain three quantum numbers (n,l,m l), which are the same as the ones obtained from .Tin is a chemical element of the periodic table with chemical symbol Sn and atomic number 50 with an atomic weight of 118.711 u and is classed as a post-transition metal. . Electron configuration chart. 1s 2: 2s 2: 2p 6: 3s 2: 3p 6: 3d 10: 4s 2: 4p 6: 4d 10: 5s 2: 5p 2: Electrons per shell: 2, 8, 18, 18, 4: Valence electrons : 4: Valency .
Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the .tin electron configuration full electron configuration of platinumThe Electron configuration of Tin is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Tin is the chemical element of the periodic table which is in group 14, its atomic number is 50 and its symbol is Sn. Tin has 10 stable isotopes. Cassiterite is its main ore. This element is obtained from the mineral cassiterite in which it occurs as tin dioxide .For hydrogen, therefore, the single electron is placed in the 1s orbital, and the electron configuration . tin: [Kr] 5s 2 4d 10 5p 2. c) lead: [Xe] 6s 2 4f 14 5d 10 6p 2. 2. Scenario: You are currently studying the element iodine and wish to use its electron distributions to aid you in your work.Electron Configuration of Tin. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (II) compounds with its two unpaired p-electrons. In the three dimensional figure below, the first and most inner electron shell is represented by blue electrons, the second electron .
The electron configuration of tin is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2. Learn how to find: Tin electron configuration. Now in the next step, start drawing the orbital diagram for tin. Draw orbital diagram. Before drawing the orbital diagram, you should know the three general rules.tin electron configuration Tin -. Sn: properties of free atoms. Tin atoms have 50 electrons and the shell structure is 2.8.18.18.4. The ground state electron configuration of ground state gaseous neutral tin is [ Kr ]. 4d10. 5s2. 5p2 and the term symbol is 3P0. Schematic electronic configuration of tin. The Kossel shell structure of tin. Solution. 1. Locate the atom on the periodic table. 2. Locate the noble gas element in the period above the element of interest. 3. Continue the electron configuration from the noble gas until you reach the element of interest. 4. Put the noble gas in brackets and write the remainder of the electron configuration.
tin electron configuration|full electron configuration of platinum
PH0 · what is zincs electron configuration
PH1 · tin electron configuration full
PH2 · sn ground state electron configuration
PH3 · full electron configuration of platinum
PH4 · electron configuration for every element
PH5 · electron configuration chart
PH6 · electron configuration calculator
PH7 · abbreviated electron configuration for tellurium
PH8 · Iba pa